Category: Legislation & Regulation
-

Plastic Microbeads
Plastic microbeads are prohibited for use in rinse-off cosmetics. If a rinse-off cosmetic contains plastic microbeads, it is adulterated (and therefore illegal).1 Key Words to Know Pastic Microbead: Any solid plastic particle that is less than 5 millimeters in size and is intended to be used to exfoliate or cleanse the human body or any part thereof.For reference, 5 mm is 0.197 inches (rounded) or…
-

FDA Warning Letters 2021
I’ve been receiving a lot notices of FDA warning letters, so I reviewed the last 106 warning letters (60 days) to see what sort of warnings they have been sending out lately. Here’s the breakdown of the types of products cited (in descending order). Unapproved New Drugs Warning letters concerning cosmetics are usually found under the “unapproved new drug” category, since that’s the most likely…
-

Making Cosmetics in Pennsylvania
Pennsylvania has requirements for cosmetic manufacturers, including registration.
-

FDA Warning Letters: Products Containing CBD
The FDA sent out 15 warning letters last week (Nov. 2019) to companies that are illegally marketing products containing CBD. I thought it might be interesting to take a look at what the FDA is going after and why. The FDA’s Position First, let’s review the FDA’s position on the marketing of products containing CBD. The FDA has acknowledged what we all know; there is…
-

FDA & Social Media
NOTE: This post is not specifically about handcrafted soap and cosmetics, but more about FDA enforcement generally. The Food Drug and Cosmetic Act defines the “label” as the written printed or graphic matter on the immediate container of a product and, and defines “labeling” as all written, printed, or graphic matter accompanying an article at any time. The FDA has authority over cosmetic, drug, food,…
-
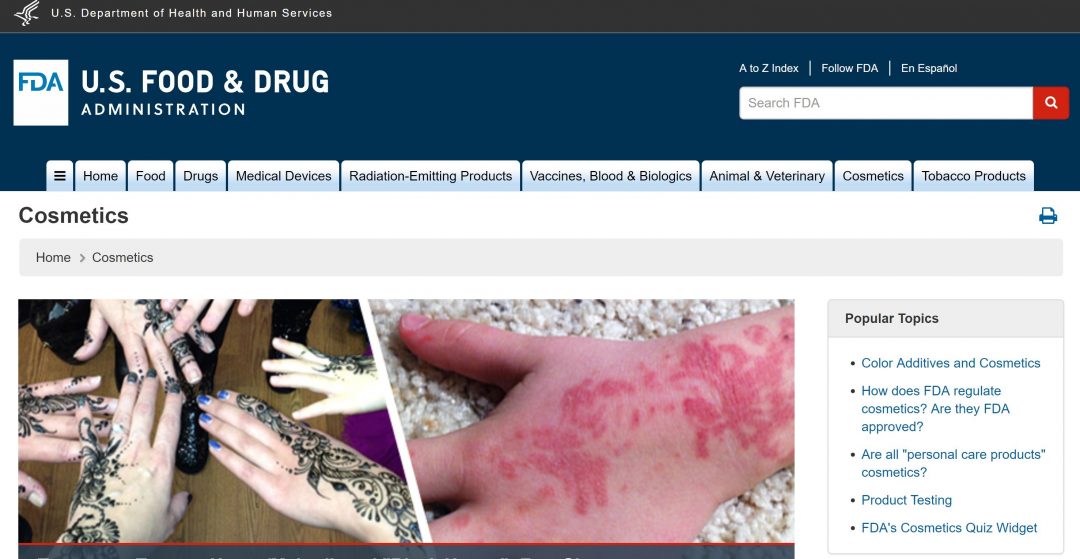
FDA Site Update
If you’ve gone to the FDA website in the past week or so, you probably noticed that their website has been updated and now looks completely different. According to their website, they are changing over to a new “more modern” WCMS (Website Content Management System), the biggest benefit of which is that it will be easy to view the site on any device. In other…
-

FDA Warning Letters Over CBD
Three FDA warning letters issued in the past week have been concerning products containing CBD. Looks like they are starting enforcement activities in this area. All three of the warning letters (to PotNetwork Holdings, Advanced Spine and Pain, and Nutra Pure) cited similar violations. Drug Claims Claims that the product would treat or mitigate various diseases, aches, and pains, including: And that’s just a sampling…
-

What about CBD Oil?
CBD is short for cannabidiol, one of the many compounds found in the cannibis plant. It is non-intoxicating, but many claim it has significant physical benefits. “Hemp” and “marijuana” have been lumped into the same category since the 1937 Marijuana Tax Act and the 1970 Controlled Substances act. However, in December the 2018 Farm Bill was passed by US Congress, and it separated out the…
-

Certificate of Free Sale
A Certificate of Free Sale is a document that provides proof that a product is being freely and legally sold without restriction. It is sometimes also called a “Certificate for Export” or “Certificate to Foreign Governments.” When Is a Certificate of Free Sale Needed? A Certificate of Free Sale is needed if you are exporting from the US to another country; but not all importing…
-

Why is Regulatory Compliance Important?
When looking at the minutiae of labeling a soap or cosmetic product, it’s easy to forget the big picture. Why are all these regulations in place? Why is it important to comply with them? Why Are There Regulations At All? In the USA, regulations are the rules that are put in place to implement the laws that are passed by Congress. When an Act is…
-

California Cosmetic Regulations Updated
Two bills concerning cosmetic manufacturing in California have been working their way through the legislative process. California Governor Jerry Brown signed AB 2775 on Sept 14th, 2018, and SB 1249 went to the Governor on September 12th. What does it mean for handcrafted cosmetics? AB 2775 – Professional Cosmetic Labeling California AB-2775 requires that all cosmetics that are “for professional use only” have the ingredient declaration…
-

Making Cosmetics in Connecticut
Connecticut requires that Cosmetic Manufacturers get licensed. Licensing requires an inspection that is primarily focused on Good Manufacturing Practices.
