Category: Soap & Cosmetic Labeling
Blog posts that deal with soap and cosmetic labeling; addition information, questions asked and answered and updates as new information becomes known.
-

Simple Labeling Checklist
It can seem like labeling is complicated. Use this checklist to see if you need to dig a little deeper to get it right … or if your label is okay as is.
-

They Do It…Why Can’t I?
“Wait! What! This other company does it, why can’t I??” Just because someone else is (wrongly) doing it, doesn’t mean that you can.
-
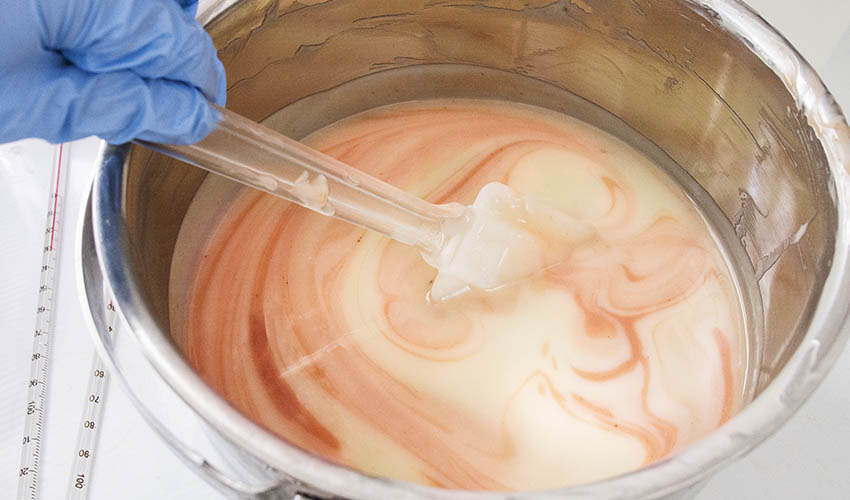
Unsaponified Oils in the Ingredient Declaration
In a soap ingredient declaration you can list what goes INTO the pot, or what comes OUT of the pot. So what do you call the unsaponified oils?
-

New Book! Navigating the Rules & Regs
New book! Navigating the Rules and Regs is finished and available at Amazon!
-

Natural Ingredients and Products
As of now (2022) the FDA has declined to provide a formal regulatory definition of the term “natural” as applied to cosmetics (or food). That said, there are some standards that can guide you concerning when the claim of “natural” is appropriate (that is, not false or deceptive) for a cosmetic product. FDA – Food While there is no FDA regulatory definition for “natural” as…
-

Talc
As an ingredient in cosmetics, talc has been under scrutiny for some years now. I’ve recently done a survey of the current information about talc, and here’s what I found out. Talc was originally used as “talcum powder”—the original body or baby powder. Now it has many uses in cosmetics and other personal care products; to absorb moisture, absorb oils, to prevent caking, to make…
-

Made in the USA
If you want to make the claim that a product is “Made in the USA” you must comply with the Federal Trade Commission’s “Made in the USA” policy.
-

The Importance of Good Manufacturing Practices
Sometimes it’s easy to think that keeping in Good Manufacturing Practices when making cosmetics is just a good idea—a “suggestion” rather than a “rule.” I suppose that idea is reinforced by the fact that cosmetic GMP isn’t actually in the regulations, it’s a “guidance.” Events of the past several months have shown that GMP failures can have devastating, even deadly, results. The story I’m about…
-

What is INCI?
The INCI is a list of the standardized and internationally accepted names of ingredients which should be used in the ingredient declaration …,. mostly.
-

FDA Policy Change on Hand Sanitizers
On October 12th, 2021, the FDA announced they are withdrawing their temporary policies allowing manufacturers who were not drug manufacturers to produce certain alcohol-based hand sanitizers. Background In the US (and elsewhere) hand sanitizers are classified as drugs—usually over-the-counter drugs. They must be manufactured by approved drug manufacturers in facilities that are registered, inspected, and approved. Drug manufacturers must follow one of several possible formulations.…
-

Ingredient Declarations for Soap (US Only)
Is an ingredient declaration required on your soaps? Let’s take a look!
-

Label Review – Trader Joe’s Foam Soap (Video)
Let’s check out the package and label of a bottle of Trader Joe’s Botanical Bounty Foaming Hand Soap!
