[Last updated 18 Mar 2024] Here’s a quick overview of what goes on the label of a soap or cosmetic products and answers to the questions that frequently come up.
Cosmetics and non-cosmetic products have the same BASIC labeling requirements, but there are some differences. See Cosmetics vs Non-Cosmetics Requirements. In the US, soap can be either a cosmetic or a non-cosmetic products. See Soap: Cosmetic or Not? and Melt & Pour Soap: Soap or Cosmetic?
What is REQUIRED on the FRONT Panel?
- Name. Your unique product name; the name that separates your lavender soap from someone else’s lavender soap.
- Identity. What is the product? (soap, lotion, bubble bath, etc.).
- Net Quantity of Contents. How much actual product is there? In both US (oz, pounds, pints, etc.) AND metric (ml, grams, liters, kilos).
FAQ: Product Name & Identity
Are there limitations on the NAME of the product?
FOR COSMETICS, the name may not include the name of one ingredient if there are two or more ingredients in the product. See What’s in a Name?
Are there limitations on the IDENTITY of the product?
FOR SOAP, the identity of the product may not include the name of one ingredient, unless that ingredient is present at a “substantial or significantly effective amount.” See Using an Ingredient Name in a Product Name for a more detailed discussion
FAQ: Net Contents
How do the NET CONTENTS need to be stated?
The statement must be in both US Customary and metric.
For solid or semi-solid products, the net contents are measured by WEIGHT and stated in ounces (oz) and grams (g). For large products, pounds (lb) and kilos (kg) would be used. Prefacing the amount with “Net Weight” or “Net wt” IS required.
For liquid products, the net contents are measured by VOLUME and stated in fluid ounces (fl oz) and milliliters (ml). Large products would be stated in quarts (qt) or gallons (gal) and liters (L). Prefacing the amount with “net contents” is NOT required.
See Net Contents – Weight and Volume
The net contents should be placed in the bottom 30% of the label, parallel to the bottom of the container, although there is an exception for very small packages.
How big does the net contents statement need to be?
The required text size is determined by the size of the principal display panel. See What is the PRINCIPAL DISPLAY PANEL?
If the principal display panel is LESS than 5 SQUARE INCHES (usually a small container of 2 oz or less), the net contents must be at least 1/16 of an inch high.
If the principal display panel is MORE than 5 SQUARE INCHES, the net contents must be at least 1/8 of an inch high.
How is the size of the net contents measured?
When the net contents contains upper AND lower case letters (such as “Fl Oz” or “Net Weight”), then the height of the lower case “o” is measured.
When the net contents are stated only in upper case letters (such as “FL OZ” or “NET WT”), then the height of the upper case “L” (or a comparable uppercase letter) is measured.
Overall, the net contents statement will be smaller if all capital (upper case) letters are used.
Where should be net contents be placed?
The net contents should be placed in the bottom 30% of the principal display panel, parallel to the bottom of the package.
If it’s a very small package, the requirement to place it in the bottom 30% of the PDP is waived.
Are there any other requirements for the net contents?
There must be clear space around the net contents. Above and below, the clear space must equal the height of an uppercase”N” in the font used. To the side, there must be clear space equal to the width of 2 “N”s in the font used.
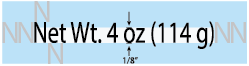
The text must be well contrasted with the background.
There cannot be any images or design that obsures or interferes with the clarity of the net contents.
FAQ: Other label front details
Can anything else go on the front?
Absolutely!
You can put marketing text on the front to tell the consumer about your product! Pictures, images, graphics, branding—those can all go on the front.
The front is a good place to put the scent or flavor of the product.
Is there anything that CAN’T go on the front?
It’s best not to put “organic” claims on the front of the package.
“Organic” claims on the front are regulated by the National Organic Program.
What is REQUIRED on the BACK or SIDE Panels?
For all products:
- The business name and address of the responsible person.
For cosmetic products:
- Ingredient declaration
- Directions for safe use (if applicable)
- Any FDA required warning statements
FAQ: Business Name
What is a RESPONSIBLE PERSON?
First off, the responsible “person” can be either a person or a company (both are considered a “person” in legal terms). It is the business that is named on the label.
The responsible person is the entity that is responsible for receiving adverse event reports from consumers and reporting them to the FDA.
The responsible person is also the entity responsible for listing the product with the FDA if they are not exempt as a small business. To be exempt as a small business, the company must have under $1 million annual revenue (average over past 3 years) AND not manufacture or sell cosmetic products that are ingested or injected, that are applied to the mucous membranes of the eye (e.g., mascara), or that stay on the body for more than 24 hours and are normally removed by a professional (not at home).1
Whose BUSINESS NAME goes on the label?
Normally it is the name and address of the manufacturer.
For a private label or contract manufactured product, it could be the name of the company that is selling the product, with the business name/address prefaced by “manufactured for” or “distributed by” to show that the company didn’t actually make the product.
What BUSINESS NAME must go on the label?
It’s the legal name of the manufacturer, or the person/company for whom the product was made.
If the business has been registered as an LLC, partnership, or corporation, you must use the official, legal name. Even if a DBA or “ficticious name” has been filed for the company, you still use the legal, official name.
If it’s a sole proprietorship, then the person’s legal name is the “business name.” However, in the case of a sole proprietor, if a DBA or ficticious name statement has been filed with the city, county, or state, you may use that as the business name on the label.
FAQ: Business Address
What ADDRESS must go on the label?
It must be the actual, physical street address of the business named on the label. It would be where the business is carried out or the product manufactured.
Is the actual street address required?
For SOAP (non-cosmetic), the street address may be omitted if it is listed in a readily accessible, widely published, and publicly available resource, including but not limited to a printed directory, electronic database, or website.1
For COSMETICS, the street address may be omitted if it is listed in a print or online phone or city directory. However, meeting the requirements for a non-cosmetic may be acceptable to the FDA.2 See Street Address – Your Choices.
The city, state and zip code are always required.
What about using a PO Box or mailing service that has a street address?
No, it must be the actual location where the business is managed or the product is manufactured.
Is there a text size requirement for the business name and address?
No. It just needs to be readable to a typical consumer under normal conditions.
FAQ: Ingredient Declaration
What order should the ingredients be listed in?
List the ingredients in descending order of predominance. In other words, the highest percentage ingredient is listed first, followed by the next highest percentage, etc.
Alternatively, you can list all the ingredients present at more than 1% in descending order of predominance, then list the ingredients present at 1% or less in any order, and then list any color additives in any order regardless of the amount.
How should the ingredients be named?
Use the name accepted in the cosmetic industry—the INCI (International Nomenclature of Cosmetic Ingredients) name.
EXCEPT – in the United States, botanical (plant-based) ingredients should NOT use the INCI name, which is the accepted scientific name for the plant.
In the US, How should botanical ingredients be named?
In the US, the FDA has stated botanical ingredients (plant-based) should be listed by their common English name. The scientific name (which is used by INCI) may be placed in parentheses.
Example: Lavender Oil OR Lavender (Lavandula Angustifolia) Oil
Is “saponified oils of …” or “saponified …” acceptable?
No, not for cosmetics. When a soap is labeled as a cosmetic, the ingredient declaration must meet the requirements, which includes using the correct name for the ingredients.
For soap, the easiest way is to list what goes INTO the pot (the oil and sodium or potassium hydroxide).
If you want to list the “reaction products” (what comes OUT of the pot), the correct name is “sodium ____ ate” (i.e. “sodium olivate” or “sodium cocoate”). In addition to the reaction products, what comes out of the pot ALSO includes glycerin and unsaponified oils (the “superfat”). Unsaponified oils can be identified as “mono- and di-glycerides” or by the glycerides of the types of oils (i.e.”cocoglycerides” or “olive glycerides”).
Are there special rules for color additives?
Color additives must be identified by the same name the FDA uses to identify them.
Dyes that are approved for use in cosmetics are identified by a color and a number (for example,”Blue 1″ or “Red 33”).
Tricky items:
- Use iron oxides (plural) for ANY of the iron oxides, regardless of the color.
- Use ultramarines (plural) for ANY of the ultramarines, regardless of the color.
- Treat colored mica as a blended ingredient and list all the component ingredients separately.
What about blended ingredients?
Blended ingredients must have all of the component listed separately, in descending order of predominance in the whole. This applies to soap or cosmetic bases as well as blended ingredients such as preservatives or emulsifiers.
Colored mica is a blended ingredient. Mica itself is translucent and doesn’t really have any color, only a little bit of sparkle. The COLOR part of colored mica comes from other components that are added to the mica. Each of the components of the colored mica should be listed separately in the ingredient declaration.
For any blended ingredient, your supplier should provide you with the information you need to create your ingredient declaration correctly.
How should infusions, extracts, or teas be listed in the ingredient declaration?
Any time you have a botanical that has been soaked in some sort of liquid solvent (such as oil, water, alcohol), it’s treated as a blended ingredient. Some components of the plant end up in the liquid and are then added to the product.
The liquid solvent is listed in the ingredient declaraton based on the amount that was added to the product. It is named by the solvent name (such as “olive oil”).
The components of the plant in the liquid are called an “extract” and are listed based on the amount of the plant material that made it into the product (usually less than 1%, so an exact amount is not necessary for determining the placement in the ingredient declaration. It is identified as an extract of the plant (such as “calendula extract”).
Is there any time an ingredient may be omitted from the ingredient declaration?
In certain circumstances, incidental ingredients may be omitted from the ingredient declaration. See What are INCIDENTAL INGREDIENTS?
There is also an exemption for trade secrets, but they must be approved by the FDA before they can be left off the ingredient declaration. The FDA has not approved any trade secret applications for many years. See Trade Secrets.
What about fragrances?
Fragrances (whether fragrance oils or essential oils) may be listed as “fragrance” without identifying all the component ingredients in the fragrance blend.
Alternately, if you know what the components are that are being used as a fragrance (such as using a blend of essential oils) you may list the components by their actual names. In the case of essential oils, they would be listed by the common English name; the scientific name could optionally be placed in parentheses. Use “oil” not “essential oil” in the ingredient name.
Example: Lavender Oil OR Lavender (Lavandula Angustifolia) Oil
Is there a minimum text size for the ingredient declaration
Yes. For most products, the ingredient declaration must be at least 1/16 of an inch in height.
However, small product packages that have less than 12 square inches of available label space on the entire package may reduce the size to 1/32 of an inch. (As a 1.5 inch cubed box has a total label area of 13.5 square inches.)
When the ingredient declaration contains upper AND lower case letters, the height of the lower case “o” is measured. When it is stated only in upper case letters, the height of the upper case “L” (or a comparable uppercase letter) is measured. Overall, the ingredient declaration will be smaller if all capital (upper case) letters are used.
What about organic ingredients?
You can identify organic ingredients with an asterisk, and then put a footnote below the ingredient declaration stating that those ingredients were/are organic.
You can also state the percentage of organic content (but only on an informational panel, not on the front of the package).
Can descriptions be included in the ingredient declaration?
Unfortunately, no.
While it’s tempting to state that the palm oil is sustainably sourced or the goat’s milk comes from your farm, those are details that should be included in marketing text or materials, not in the ingredient declaration.
What about fragrance allergens?
In the US, fragrance allergens are not yet required to be identified in the ingredient declaration. In accordance with the Modernization of Cosmetic Regulations Act of 2022 (MoCRA), it will be required soon, but the regulation is not in place yet.
Proposed regulations are supposed to be issued in June, 2024, but probably won’t go into effect until sometime in 2026 or later.
FAQ: Directions for Safe Use
Are directions always required?
Not always. However, if there is something the consumer should know to use the product safely, that must be included on the package.
What sorts of things should be included in the directions?
How to apply the product. How often to use it. How to store it.
If the product might normally separate or change the way it looks, that could be included so the customer is not surprised or think something is wrong with the product.
FAQ: Warning Statements
What products REQUIRE warning labels?
Warning statements are required on:
- Bubble bath
- Feminine spray deodorant products (including some yoni products)
- Suntanning preparations that do not contain sunscreen
- Products containing alpha or beta hydroxy acids
Each different product has a specifically worded warning statement.
Where should the warning statement be placed?
The warning statement may be placed on the back or sides of the product.
Are there text size or other requirements for a warning statement?
The text must be prominent and conspicuous. It must be at least 1/16 of an inch high. See Measuring Text Sizes For Labels
Again, you can use the side and back panels for additional marketing text touting the wonderfulness of your products and why a consumer should buy them. Remember not to use any claims that could be construed as “drug” claims (see blog posts FDA Cracking Down on Cosmetic Product Claims and More on Product Claims for more info).
What other information is ALLOWED on the label?
Your package can include any type of additional marketing text touting the wonderfulness of your product and why a consumer should buy it! You can include pictures and graphic design. You can also include:
- Website address
- Phone number
- Social media addresses (or QR code)
- Batch number
- Best-if-used-by date
- Use-within-opening time
- Bar code
Other information can be included on the label, but this type of information also come with some regulatory requirements and/or limitations:
- Claims to treat or prevent disease or claims that the product will alter the function or structure of the body. See What is a DRUG?
- “Recycle” statements or logos
- Environmental “Green” claims
- “Free-Of” statements
- “Made in the USA” (or Made in a US state). See: Made in the USA and Made in “My State” Claims.
If you have a question that isn’t answered here, please feel free to email me. This information (and much more!) is contained in my Soap & Cosmetic Labeling and Navigating the Rules & Regs books.

Shameless plug!
To really be able to create your own labels that comply with the regulations, get my book from Amazon and use it.
4th Edition – Released March 5, 2025!!!
Or order directly from me (and get a signed copy)!
